Impressive Tips About How To Write A Ionic Equation
Pptx, 1.63 mb.
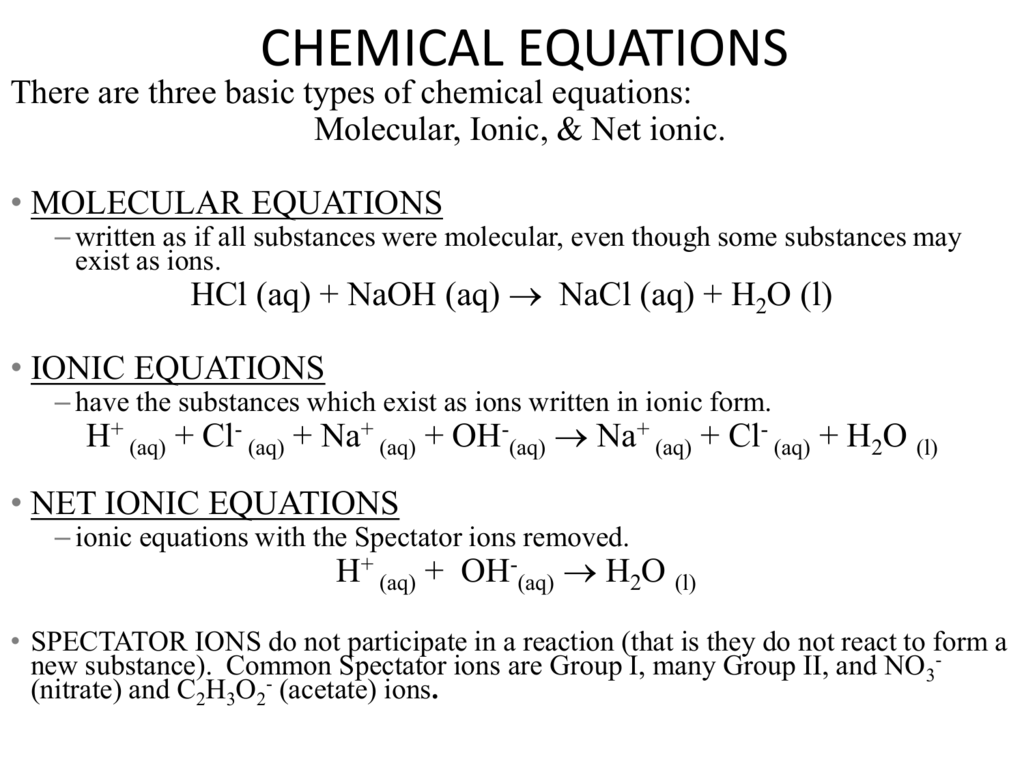
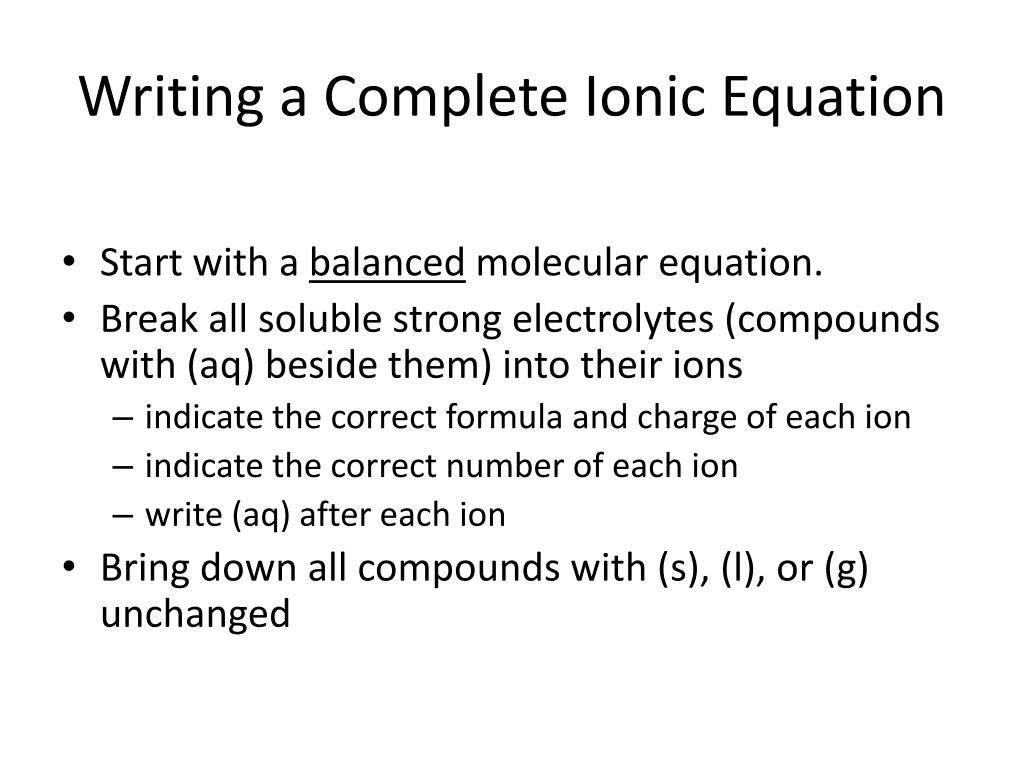
How to write a ionic equation. To obtain the complete ionic. There are three steps to writing a net ionic equation: Then write the ionic equation, showing all aqueous substances as ions.
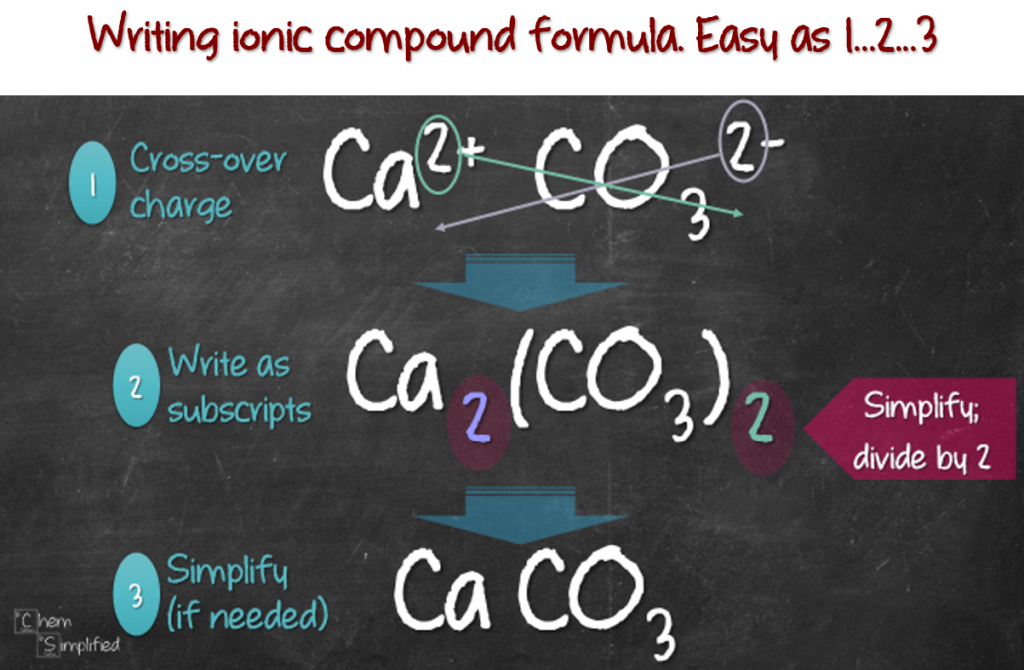
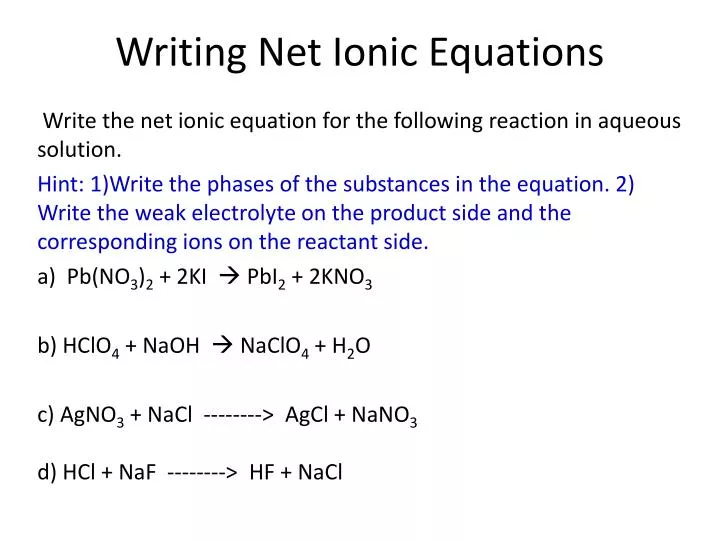
Write the correct formula for an ionic compound. Writing a net ionic equation. This video covers, how to predict products, how to balance a chemical equation, how to identify the solubility of a compound, how to write a complete ionic.
Solubility rules are very useful in. Writing ionic equation is extremely similar to writing chemical equations. Calculate net ionic equation.
Recall that ionic compounds that dissolved in water will dissociate completely into ions. Kirsten wordeman view bio. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions.
A complete ionic equation is a chemical equation in. Therefore, the formula is: Mainly for year 11 but could be useful for year 12.
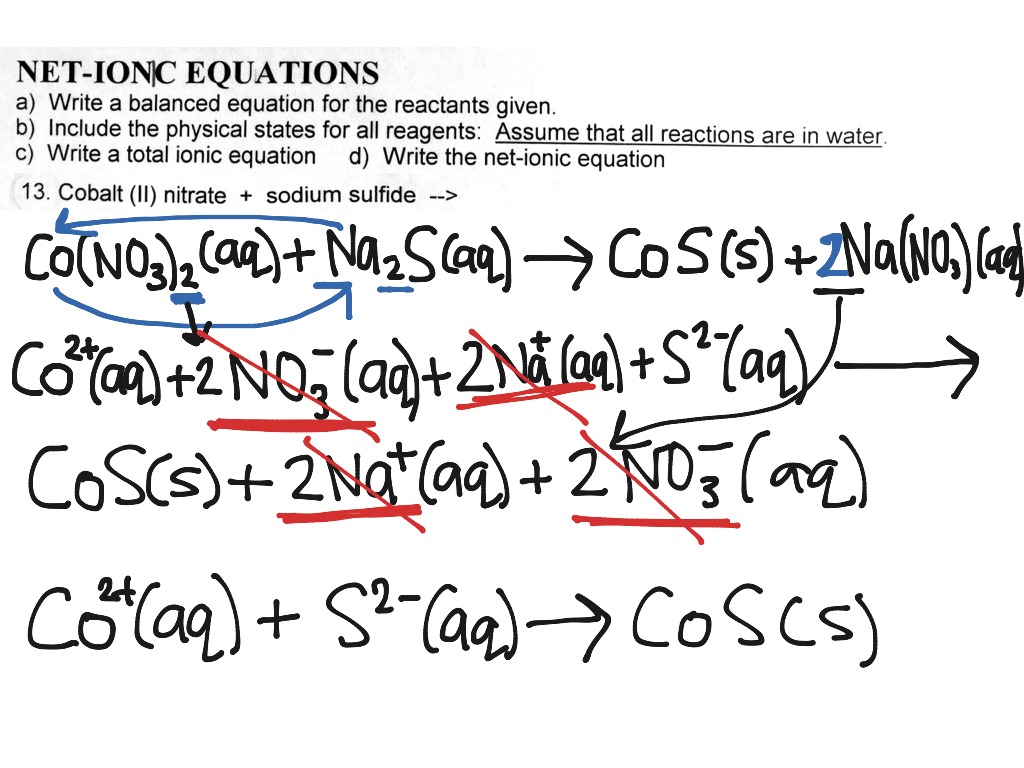
Ionic equations can be written for any reaction involving aqueous substances (ions in a solution). We know that fe (iron) has a 3 + charge. Ionic equations are similar to ordinary chemical equations, but in this case,.
This chemistry video tutorial explains how to write net ionic equations. There are three basic steps to writing a net ionic equation: The balanced equation will be calculated along.
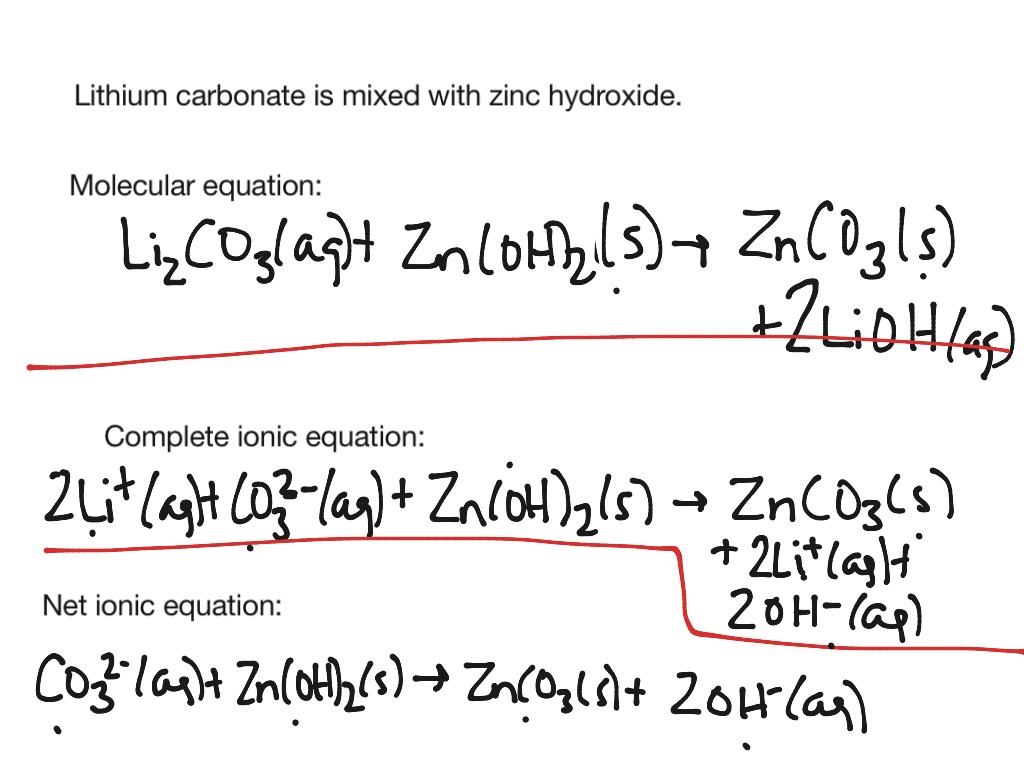
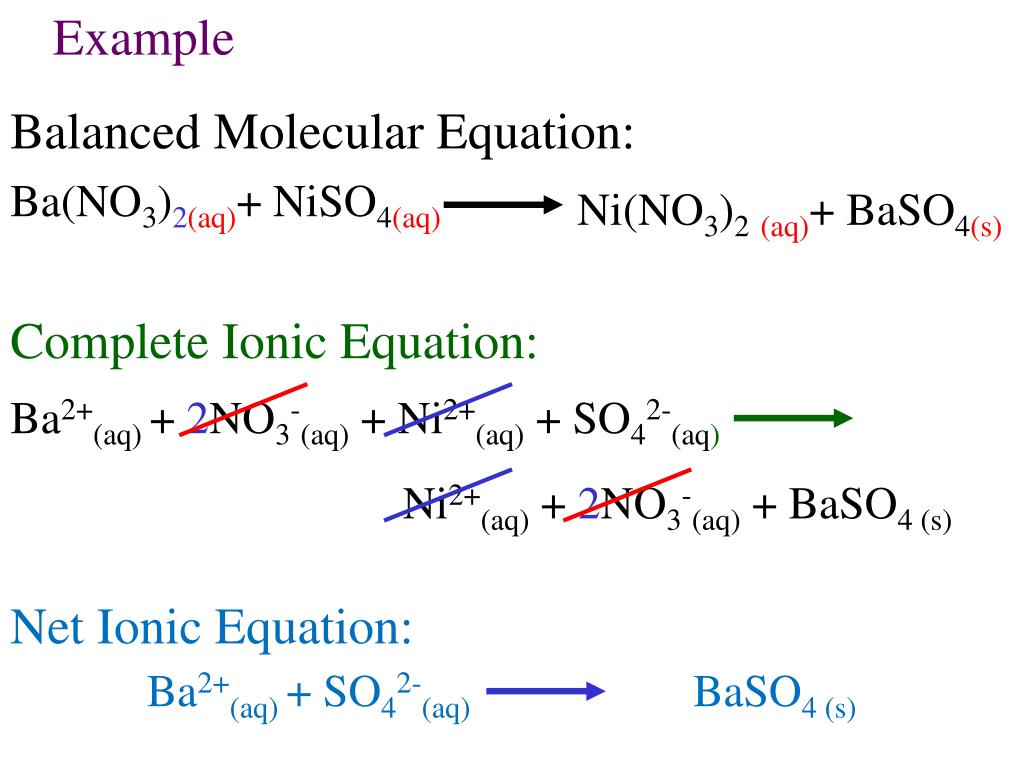
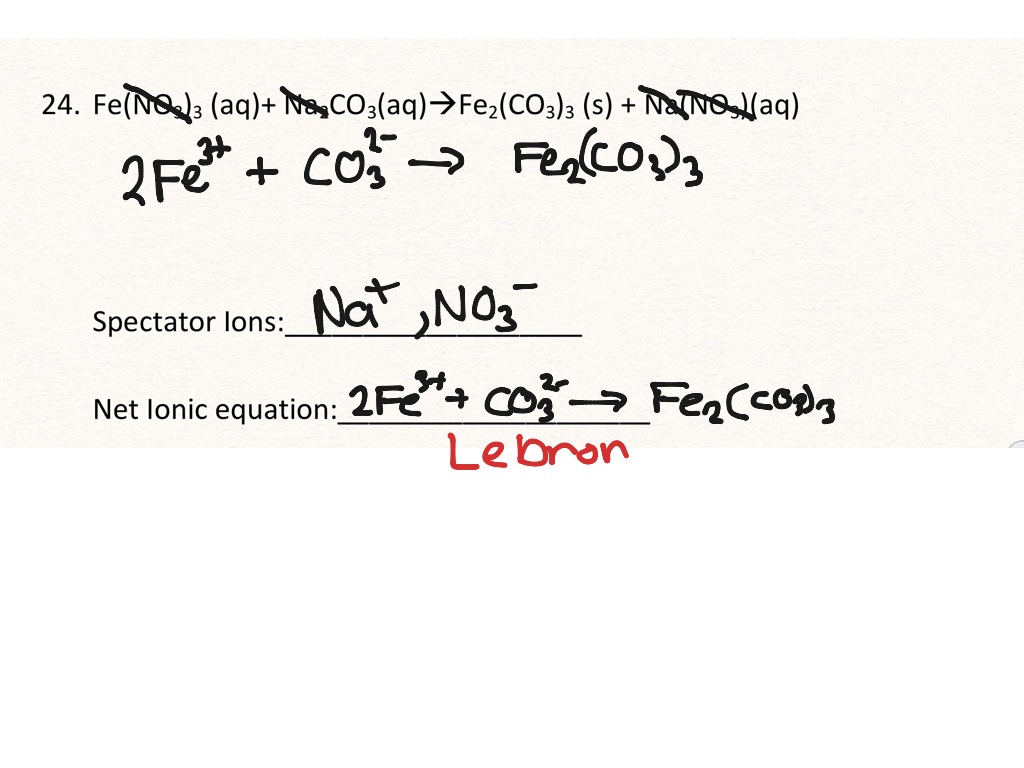
This is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. Write and balance the molecular equation first, making sure that all formulas are correct. The elemental metals (magnesium on the reactant side, copper on the product side) are.
It explains how to predict the products of double replacement reactions and acid base. Recognize polyatomic ions in chemical formulas. In the net ionic equation, any ions that do not participate in the reaction.
Write the equation in terms of all of the ions in the solution. A step by step guide on how to write ionic equations. Look at the molecular equation and identify which species are in the aqueous state.